Easy tricks to calculate equilibrium constant based problems/Chemical eq... | Simple tricks, Equilibrium, Problem
How to Calculate Equilibrium Constant K Value Practice Problems & Exampled Explained Step by Step - YouTube
![SOLVED: For the reaction A(g) B(g) + C(g) = D(g) The following data were obtained at constant temperature: Initial [A] Initial [B Initial [C] Experiment Initial Rate (moLL-S (moll) (moLL) (moLL) 0.0500 SOLVED: For the reaction A(g) B(g) + C(g) = D(g) The following data were obtained at constant temperature: Initial [A] Initial [B Initial [C] Experiment Initial Rate (moLL-S (moll) (moLL) (moLL) 0.0500](https://cdn.numerade.com/ask_images/0dd365b89cba44fdb030a3ecfdcc1920.jpg)
SOLVED: For the reaction A(g) B(g) + C(g) = D(g) The following data were obtained at constant temperature: Initial [A] Initial [B Initial [C] Experiment Initial Rate (moLL-S (moll) (moLL) (moLL) 0.0500

6-3:Calculating Equilibrium Constants: The actual value of K eq is found experimentally. The individual concentrations of all the reactants is calculated, - ppt download
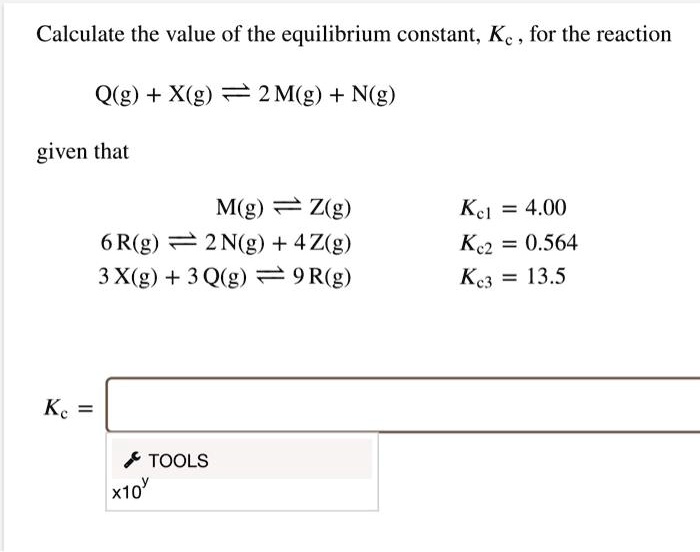
SOLVED: Calculate the value of the equilibrium constant; Kc , for the reaction Q(g) + X(g) = 2 M(g) + Ng) given that M(g) = Z(g) 6 R(g) 2 N(g) + 4Z(g)

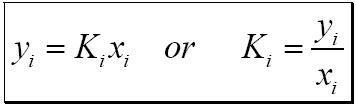

![The constant K used to calculate corrosion rate in eq. 1 [15] | Download Scientific Diagram The constant K used to calculate corrosion rate in eq. 1 [15] | Download Scientific Diagram](https://www.researchgate.net/publication/352980107/figure/tbl2/AS:1041875033145347@1625413773912/The-constant-K-used-to-calculate-corrosion-rate-in-eq-1-15.png)





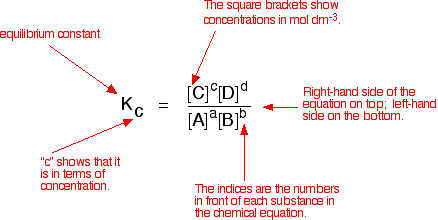


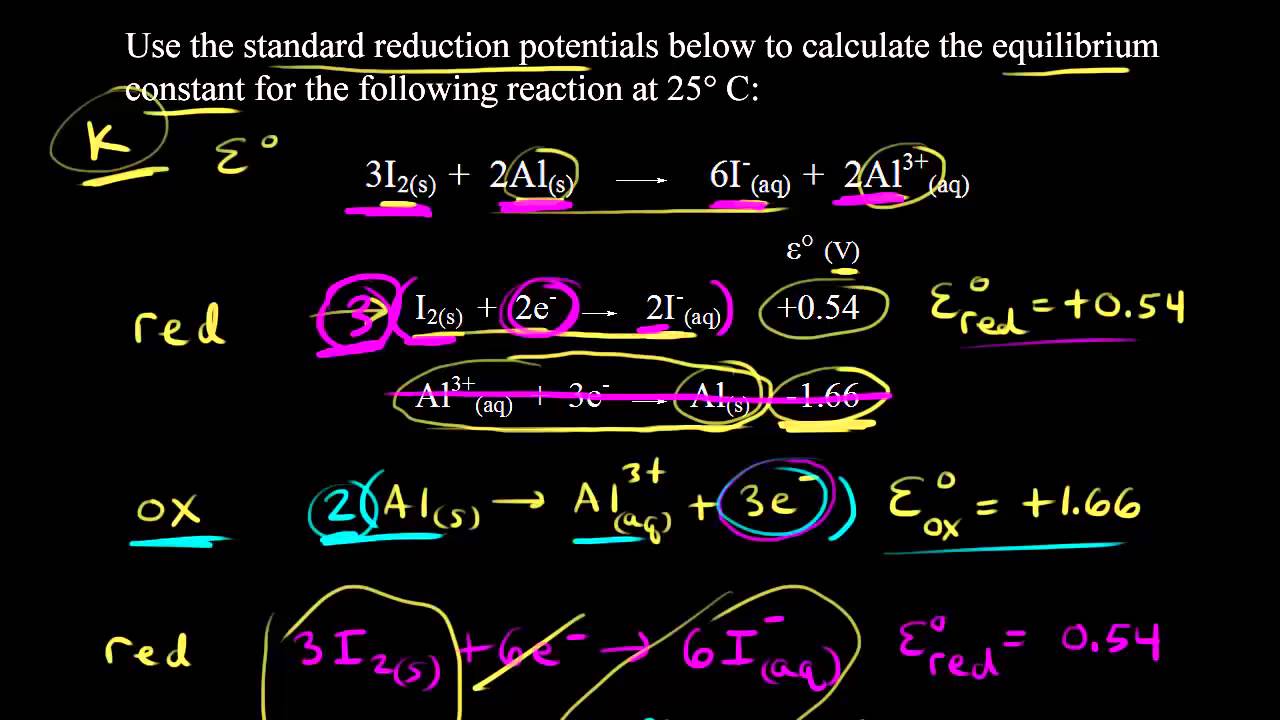
![Calculate K from [EQUILIBRIUM] concentrations 2018 - YouTube Calculate K from [EQUILIBRIUM] concentrations 2018 - YouTube](https://i.ytimg.com/vi/8l1HCaVLgVI/maxresdefault.jpg)