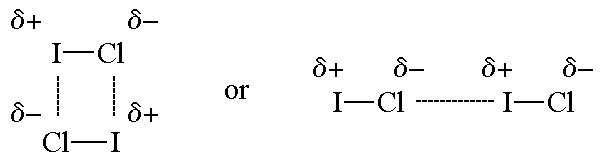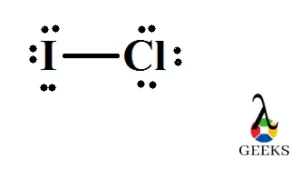Scheme 1 Conditions and reagents: (i) ICl, THF, À78 1C, 45 min; (ii)... | Download Scientific Diagram

Wild type and ICL-less (BP) cells: respective localisations of ICL1e,... | Download Scientific Diagram

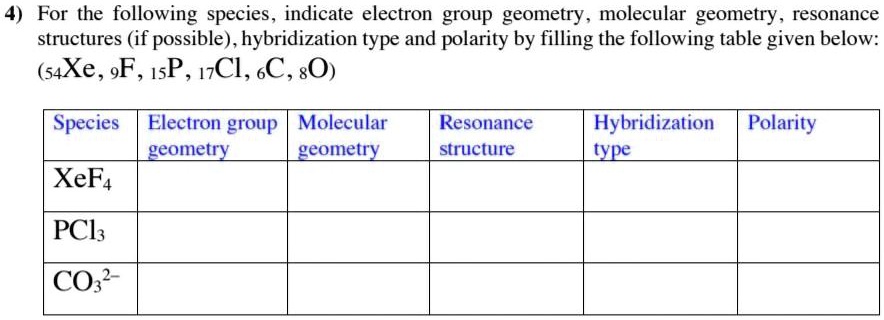
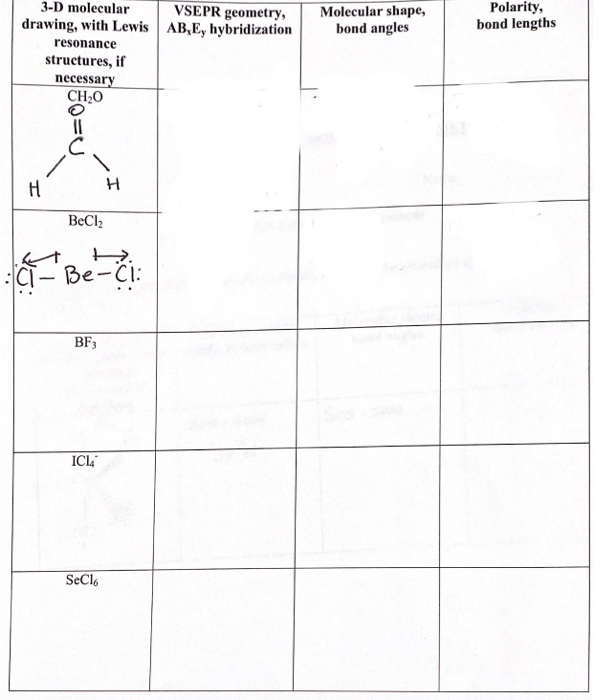
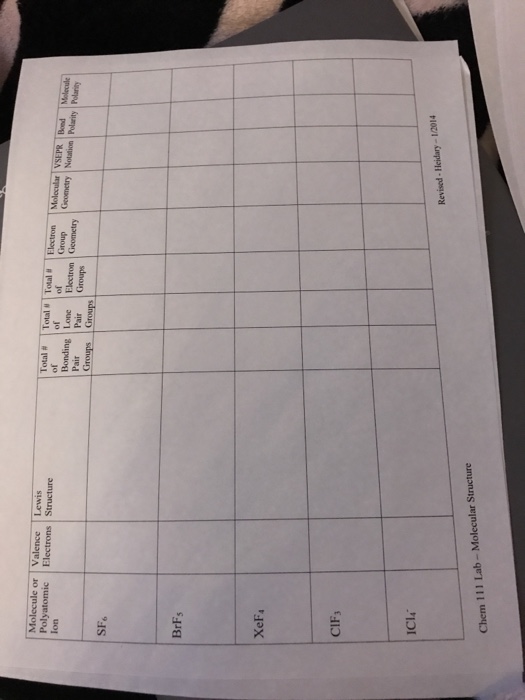
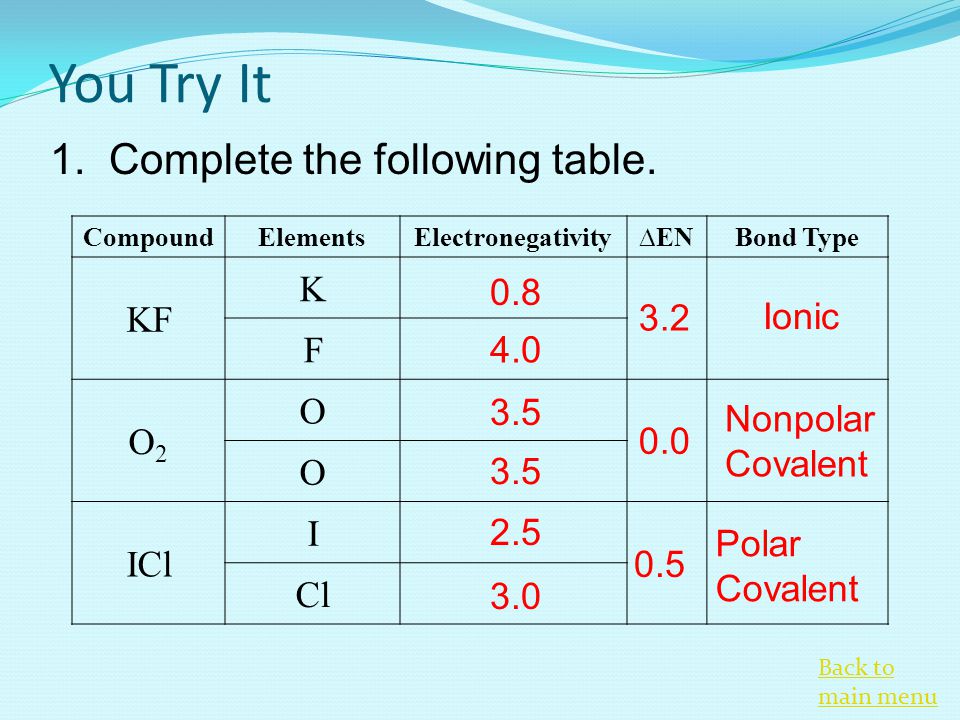
Why is ICl_{2}^{-} a non-polar molecule whereas ICl_{2}^{+} is a polar molecule? | Homework.Study.com
![ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download](https://images.slideplayer.com/34/10194070/slides/slide_3.jpg)
ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download
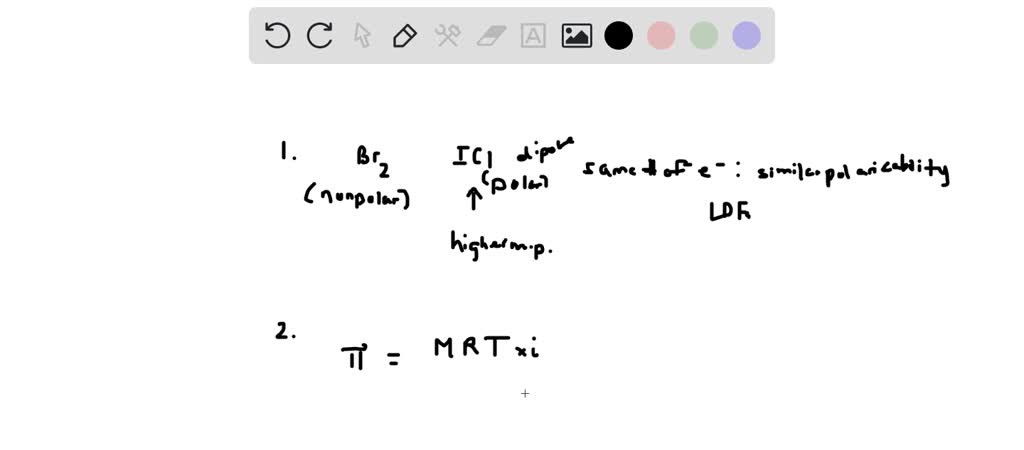
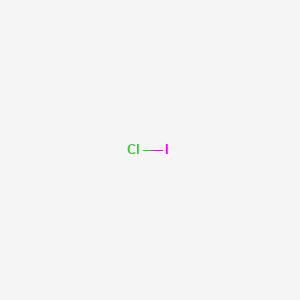
SOLVED: 1. The compounds Br2 and ICl have the same number of electrons, yet Br2 melts at -7.2°C, whereas ICl melts at 27.2°C. Explain why. 2. calculate the osmotic pressure of a

The molecular size of ICl and Br2 is approximately the same, but b.p. if ICl is about 40^oC higher that that of Br2 It is because :

Among the following the total number of polar molecules are. Cl2, OCl, BF3, NO, SO2, XeF4, H2CCl2, OCS .

a) Draw the Lewis structure for ICl_{4}^{-} b) What is the electron pair geometry for I in ICl_{4}^{-}? c) What is the shape (molecular geometry) of ICl_{4}^{-}? | Homework.Study.com